Hypothesis-Driven R&D: An idea whose time has come
Six years ago I worked with a group of biopharma managers who wanted to reduce the risk of late-stage failures. It’s time to share again what we learned, in light of the recent Phase 3 failure of aducanumad, an Alzheimer’s drug based on a clinical hypothesis that years of data had already eroded.
The article From Phase-Driven to Hypothesis-Driven R&D shows R&D leaders that rigorous work on human biology in early development reduces the risk of clinical failure.
Rather than focusing R&D programs on advancing a compound, groups can focus on advancing knowledge about how to deliver health benefits through improving human biological function. Advancing drug candidates would be the by-product of successful work, not the product.
They can visualize R&D as a series of experimental cycles:
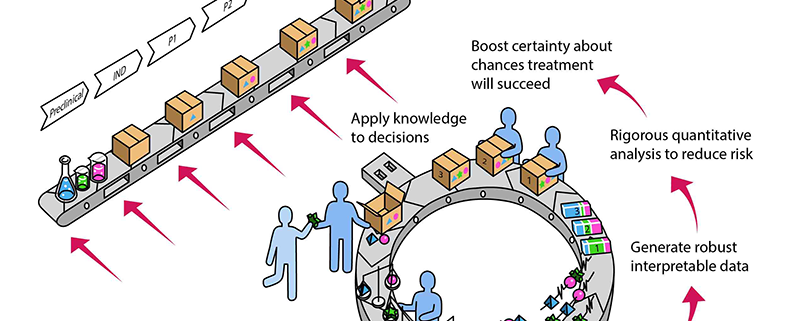
- Build hypotheses about chances that treatment will succeed
- Design sound experiments
- Execute experiments with tools fit-for-purpose
- Generate robust, interpretable data
- Rigorous quantitative analysis to reduce risk
- Boost certainty about chances treatment will succeed
- Apply knowledge to decisions.
Many of the managers I worked with then are now leading their own R&D groups, and I have the privilege of introducing these ideas to innovative startups. To learn how you can benefit, drop me a line at mkummer@kummerconsulting.com.